Abstract
Background: AMV564 is a novel bivalent, bispecific (2x2) CD33/CD3 targeted immunotherapy that binds both CD33 and the invariant CD3ε on T-cell receptors with strong avidity, thus creating an immune synapse between CD33-expressing cells and T cells, initiating T-cell directed lysis of CD33 expressing cells, and inducing expansion, differentiation and proliferation of T cells. AMV564 is being evaluated in clinical trials for patients with acute myeloid leukemia (AML, NCT03144245). Previously, we demonstrated that the parent molecule, T564, had specific, T-cell mediated cytotoxic activity against a KG-1 CD33+ cell line in vitro and eliminated blasts in an autologous AML patient-derived xenograft mouse model (Eissenberg, et al. 2015 ASCO). Here we investigated factors that may contribute to antileukemic activity of AMV564 in another, more aggressive, preclinical model of AML.
Methods: Disseminated activity studies in NOD scid gamma mice (NSG) were performed by injecting MOLM13 AML cells transduced with click beetle red luciferase and green fluorescent protein (MOLM13-CG) in the tail vein. In a series of experiments, AMV564-mediated clearance of MOLM13-CG cells and overall mouse survival was determined. Tumor cells (either 3.3 x 103 or 1 x 105) were engrafted in non-conditioned NSG mice for 3-7 days. Variables included dose of AMV564 (0.5 to 25 mcg, i.v.), number of AMV564 cycles (1 or 2) and cycle length (4-5 days), number of T cell injections (1 or 2), and total number of human T cells administered (0.2 to 1.2 x 107). Tumor burden was serially measured by bioluminescence (BLI) and survival measured for 51 days.
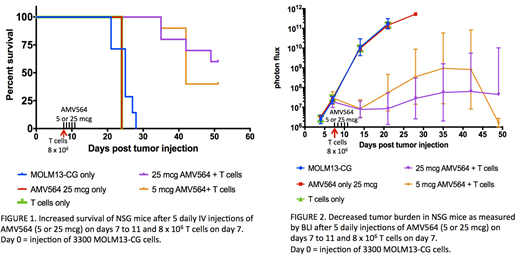
Results: Mouse survival was greatest when AMV564 was injected along with a total number of T cells ≥ 8 x 106 regardless of the number of T cell injections or their timing relative to tumor injection. AMV564 mediated up to a 3-fold increase in survival time compared to untreated mice. In the experiment shown in Figure 1, untreated mice died at day 28, yet about half of the treated mice were still alive when the experiment was terminated on day 51. In the absence of AMV564 (i.e., T cell treatment only), animal survival and tumor burden were similar to that of untreated animals.
In the same experiment we administered, AMV564 daily from days 7-11 after MOLM13-CG infusion along with one injection of 8 x 106 human T cells on day 7, then measured tumor burden by BLI signal (Figure 2). By day 14 we observed a median 4.0 log or 4.8 log reduction in tumor using 5 or 25 mcg AMV564. In fact by day 14, 4 of 10 mice treated with 25 mcg AMV564 and 2 of 10 treated with 5 mcg had no detectable signal above background. No rebound in tumor burden occurred in these mice throughout the 51-day study.
Conclusions: AMV564 is a potent and effective immunotherapy against an aggressive human AML cell line in NSG mice. AMV564 very effectively prolonged survival and dramatically reduced tumor in the bone marrow and peripheral blood. These results are consistent with our previous preclinical data with primary AML and support the testing of this reagent in patients with relapsed and refractory AML in the clinic.
Eissenberg:Amphivena Therapeutics: Research Funding; Novimmune: Research Funding. Rettig:Novimmune: Research Funding; Amphivena Therapeutics: Research Funding. Fox:Sunesis Pharmaceuticals: Employment; Amphivena Therapeutics: Employment. Guenot:Amphivena Therapeutics, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.